There are so many branded detergent in the store but how do we determine which brand are the most effective brand?
Chlorine is added to the detergent to act as bleaching agent. Chlorine able to displace iodine as the chlorine is a stronger oxidising agent compare to iodine. So, we can use iodine to titrate with known concentration of sodium thiosulphate to determine the concentration of the’ free chlorine’ in the detergent in order to compare which work the most effective. The more the concentrate of ‘free chlorine’, the more effective the detergent works.
How detergent work?
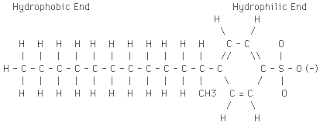
We have to understand the structure of the detergent molecule in order to know how the detergent works. The detergent molecule consists of two parts hydrophilic head and hydrophobic tail. Hydrophilic head is made up of sulphonate group which will attract the water molecule to be bind with it as it is polar. While, the tail is made up of a long hydrophobic hydrogen carbon tail.
In water, the hydrophilic head which is negatively charge will dissolve in water, as the water is positively charge. The hydrophobic tail will dissolve on the dirt layer. So, when the water is stir the grease will lift off from the surface. The dirt is emulsify after being lift off from the surface by the surfactant. The dirt then will be breaks into small pieces and wash away with water. They will not clump back into large molecule because each of the small molecule are carrying the same charge, negative charge they will repeal each other.
Do you know?
There are two types of water soft and hard water. Hard water contain high amount of mineral with it especially with Mg2+and Ca2+ ions. While the soft water is free of any minerals. Detergent is effective in both soft and hard water as it do not form scum with Mg2+and Ca2+ ions.

No comments:
Post a Comment