- What is condoms actually?
- What does it for?
- How does it works?
- What is condoms made of?
There are many questions over many children's mind.
How Does It Work?
Condoms work by keeping semen (the fluid that contains sperm) from entering the vagina. The male condom is placed on a guy's penis when it becomes erect (and before any sexual contact). It is unrolled all the way to the base of the penis while holding the tip of the condom to leave some extra room at the end. This creates a space for semen after ejaculation and makes it less likely that the condom will break.
The two main function of condoms basically is:
- Prevent from pregnancy
- Stop spreading sexually transmitted diseases (i.e: HIV)
Latex condoms
- With oil-based lubricant.
- likely to slip off due to loss of elasticity caused by the oils.
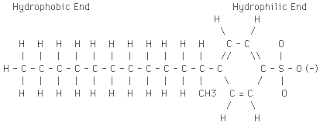
Latex(rubber) structure
Polyurethane condoms
- Same width and thickness as latex condoms.
- 0.04 mm and 0.07 mm thick.
- Material of many female condoms.
Lambskin condoms
- Made from lamb intestines.
- Greater ability to transmit body warmth and tactile sensatio.
- Cause fewer allergenic reactions than latex.
- Increased risk of transmitting STDs compared to latex because of pores in the material, are large enough to allow infectious agents to pass through.
All condoms are more comfortable for both partners when use with additional or latex-safe lubricants.
Lubricants reduce friction during sexual intercourse, which lessen the risks of breakage by keeping the barrier moist and in doing so, also help ensure that genitals remain moist enough to make barrier use more pleasurable.
Nonoxynol-9
- N-9.
- Non-ionic nonoxynol surfactantthat used to cleaning and cosmetic products.
- Widely used in contraceptives for its spermicidal properties.
- Active ingredient in most spermicidal creams, jellies, foams, gel, film, and suppositories.
Below are the examples of types of one of the famous brand for condoms (durex)
Regular condoms for a natural feeling
Narrower condom to provide a tighter fit for extra confidence.
Our thinnest latex condom for ultimate sensitivity
It’s the special lube that creates the gentle tingling.
Longer and wider for a better fit!
Three fun fruit flavours.
Ultra fine for enhanced sensitivity.
500 unique raised dots mean 100% enhanced pleasure.
It’s the one with uniquely positioned ribs and raised dots. In all the right places!
With a gentle lubricant for a natural feel during lovemaking.
Slightly thicker. With extra lubrication. For those who want the ultimate reassurance.
An exciting selection of condoms and lubes.
Non-latex condoms – suitable for sensitive users.